Introduction
In decorative coatings the use of binder-free, very compatible pigment concentrates is a flexible way to produce colored paints. The highest volume are concentrates based on iron oxides, which need to meet high demands in storage stability, color strength and cost efficiency.
Different structures of wetting and dispersing additives can be used in waterborne, binder-free pigment concentrates for iron oxides. Their performance is focused on the stability and the wetting of the pigments which is reflected by the viscosity reduction, the storage stability of the pigment concentrates and the coloristic properties. For the characterization of the wetting behavior and the dispersing efficiency, the Zeta potential can be used.
Pigment dispersion process
In the first step of the dispersing process, the surface of the pigments has to be wetted by a liquid. The air in the pigment powder has to be replaced by water and dispersing additive. The viscosity reduction in the grinding stage is a first indication of successful pigment wetting. [1]
The target of the dispersing process is to achieve very small particle sizes with a large surface area which leads to higher color strength and good hiding power. During the second step, the grinding stage, the pigment agglomerates are broken down mechanically ideally to primary particles.
The third step of the dispersion process is the stabilization.
The stabilization of the particles can be carried out mainly by electrostatic and steric stabilization.
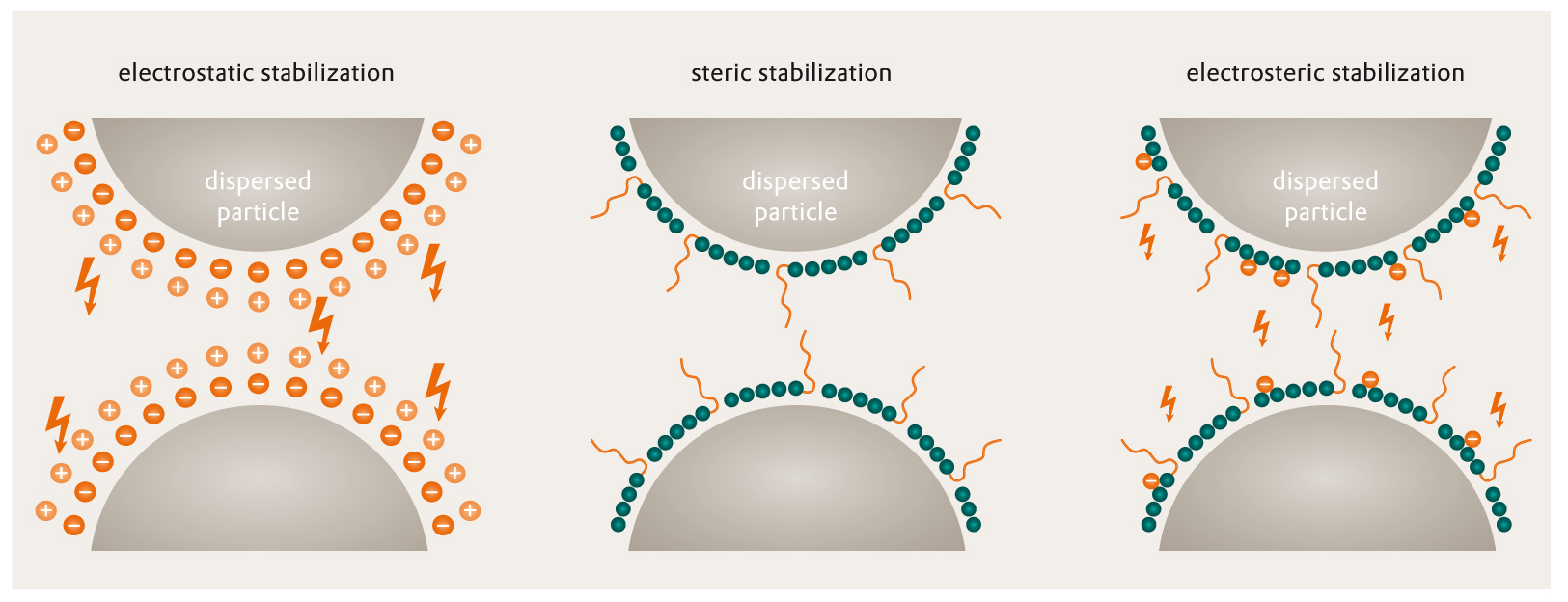 Picture 1: Mechanisms of Pigment Stabilization [4]
Picture 1: Mechanisms of Pigment Stabilization [4]In case of electrostatic stabilization, the dispersing additive dissociates into anions adsorbed on the pigment surface and cations, that form a mobile diffuse cloud around the particle. The electrostatic double layer stabilizes the particles against flocculation by repulsion.
Electrostatic stabilization - Zeta potential
The effectiveness of the electrostatic stabilization can be described by the zeta potential ζ. In general, it describes the potential between the electrostatic double layer caused by ionic dispersing additives. [2]
It can be measured e.g. electro acoustically.
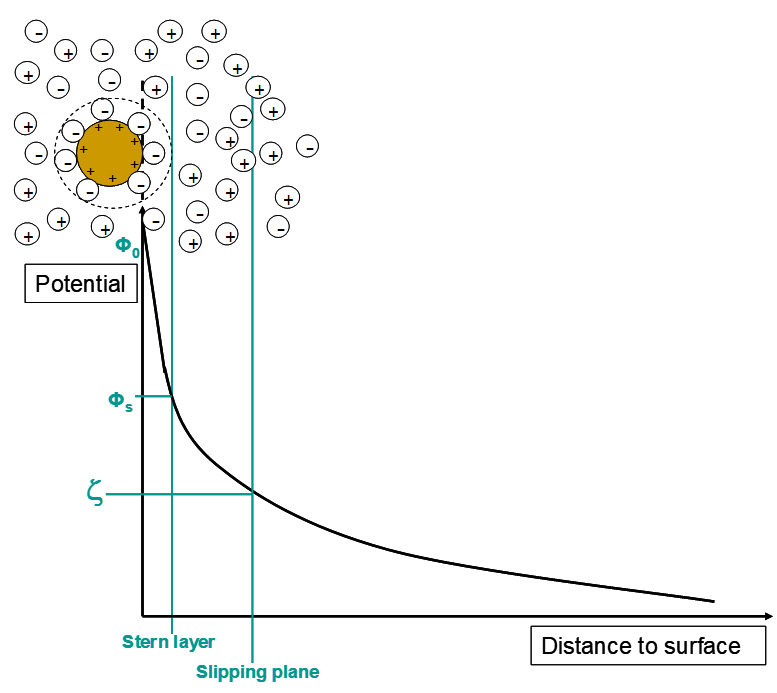 Picture 2: Mode of Electrostatic Stabilization
Picture 2: Mode of Electrostatic StabilizationBy titrations with additive the interactions between pigments and additives can be characterized and so the electrostatic stabilization can be described.
The higher the zeta potential the better the stabilization of the pigments.
The zeta potential does not describe the steric stabilization which is another important mechanism in waterborne formulations. The steric stabilization is not achieved by ions and so no potential can be measured.
Steric stabilization
To create steric stabilization the additive contains polymeric side chains, that must be soluble in the surrounding medium. The overlap of polymeric side chains of different particles restrict the freedom of movability, which leads to a lower entropy level and thereby induces an energy barrier between the particles.
Discussion of the results
Typical formulations of waterborne binder-free pigment concentrates for iron oxides were used to prepare pigment concentrates. Three different polyacrylate salts, one highly polymeric additive and a new additive have been tested.
|
|
PY 42
|
PR 101
|
Water
|
38.4
|
32.9
|
Dispersant
|
5.5
|
6.0
|
Pigment
|
55.0
|
60.0
|
Biocide
|
0.1
|
0.1
|
Defoamer
|
1.0
|
1.0
|
Additive solid on pigment
|
10%
|
10%
|
Table 1: Tested Formulations
Particle size and hiding power
The highest hiding power of particles is achieved when the particle size is half of the wavelength of the light (λ/2). For iron oxide yellow the optimum is at about 350 nm.
With the new additive the particle size of iron oxide yellow was close to this optimum.
Additive
|
Particle size MI [nm]
|
Δ E
|
New additive
|
316
|
18.08
|
Polyacrylate
|
530
|
19.65
|
Table 2: Particle Size and Hiding Power
Viscosity and stability
The viscosity of the pigment concentrates was measured 24h after preparation and after storage for two weeks at 50°C with a cone plate rheometer.
The polyacrylate salts showed useful results with both PY 42 grades. The polymeric additive and the new additive exhibited a stronger viscosity reduction. One of the polyacrylates did not give a stable viscosity over time. The other additives showed stable viscosities without settling.
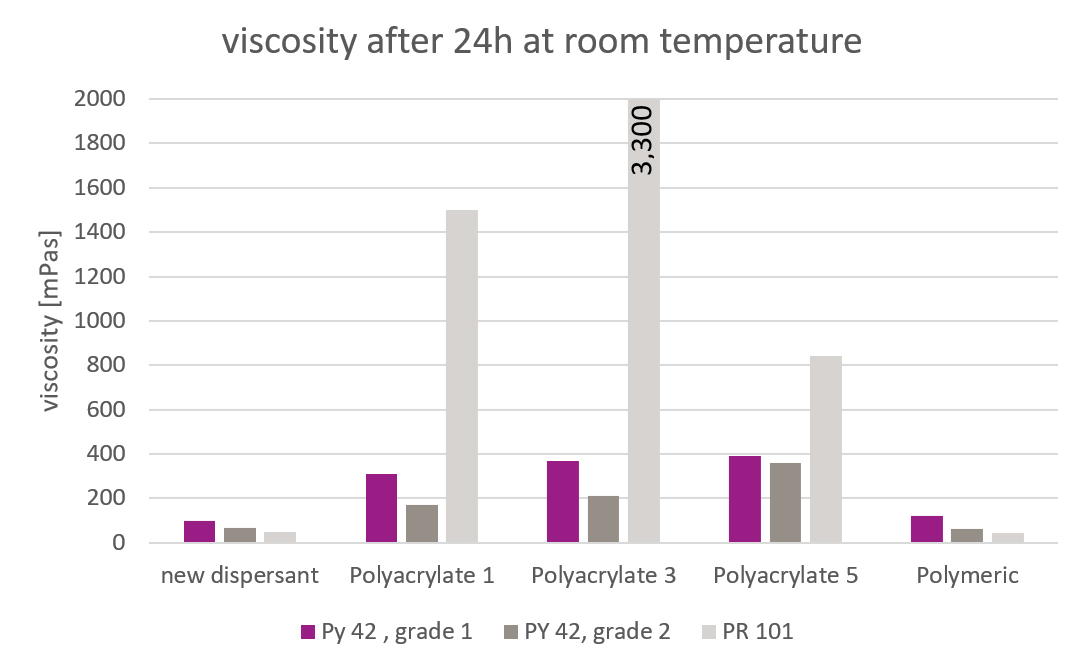 Picture 3: Initial Viscosities of the Pigment Concentrates
Picture 3: Initial Viscosities of the Pigment ConcentratesZeta potential
The zeta potential was measured in a 5% pigment slurry. The respective additive was titrated until a constant zeta potential was reached. The lowest zeta potential was achieved with polyacrylates. This chemistry gives the strongest electrostatic stabilization. The non-ionic highly polymeric additive had almost no influence on the zeta potential. It provides no contribution to electrostatic stabilization. The zeta potential achieved by the new additive was in between the two others.
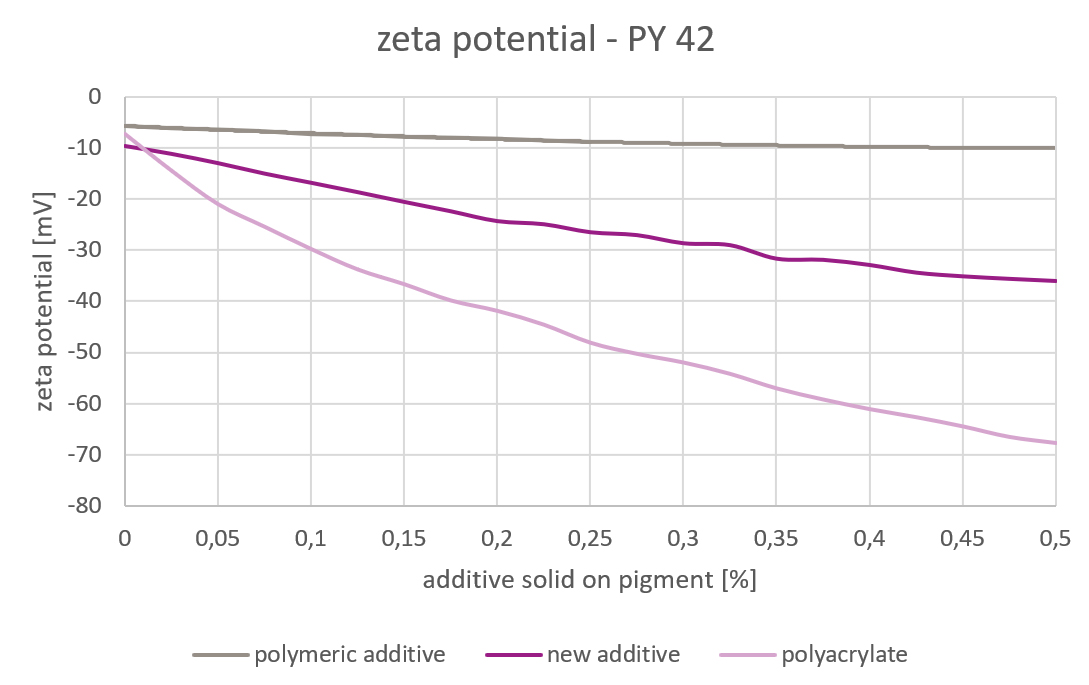 Picture 4: Zeta Potential – Iron Oxide Yellow
Picture 4: Zeta Potential – Iron Oxide YellowIt is obvious that the zeta potential is not sufficient to interpret the results obtained by viscosity and stability measurement. The zeta potential does not give information about the steric stabilization.
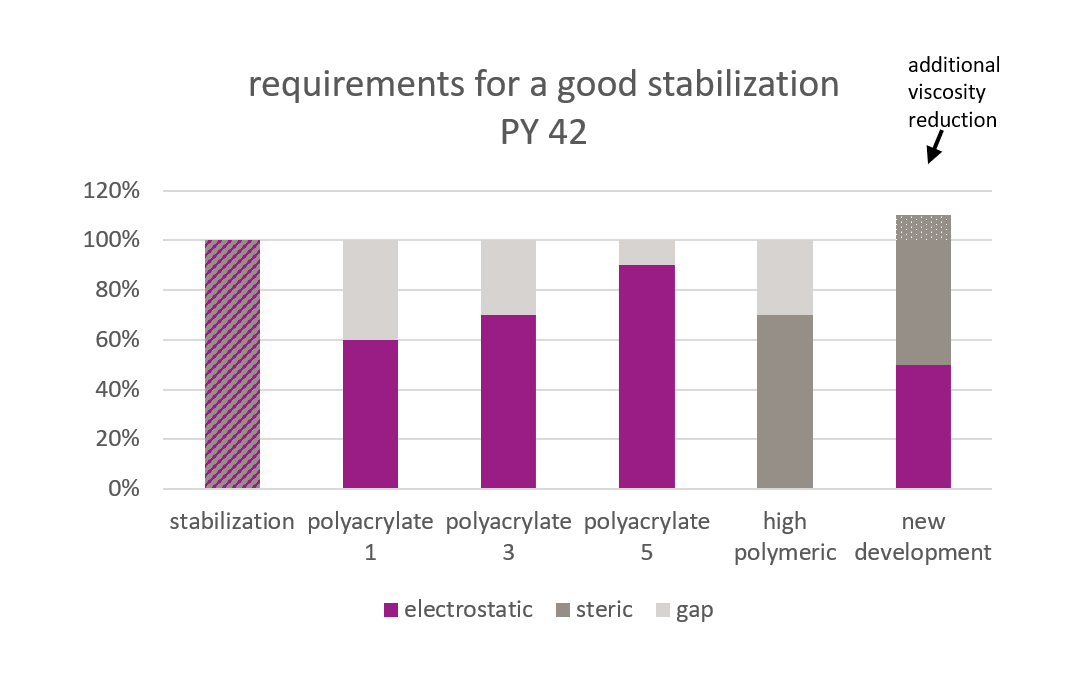 Picture 5: Stabilization of PY 42
Picture 5: Stabilization of PY 42To stabilize the iron oxide particles, a certain amount of stabilization energy is needed. Not in all cases can this amount of energy be provided by electrostatic energy alone. For those cases, additional steric stabilization is necessary.
Conclusion
The results show that the new additive performs on a very high level with a broad range of iron oxide grades. The new additive provides an optimized balance between electrostatic and steric stabilization making it a very efficient additive.
Authors
Frank Kleinsteinberg, Markus Vogel
Evonik Operations GmbH
Literature
[1] Additives for Waterborne Coatings – Wernfried Heilen et al.
[2] Dispergieren von Pigmenten und Füllstoffen – Jochen Winkler