This innovation represents a significant step forward in tackling PFAS pollution and advancing safer, more efficient pharmaceutical manufacturing methods.
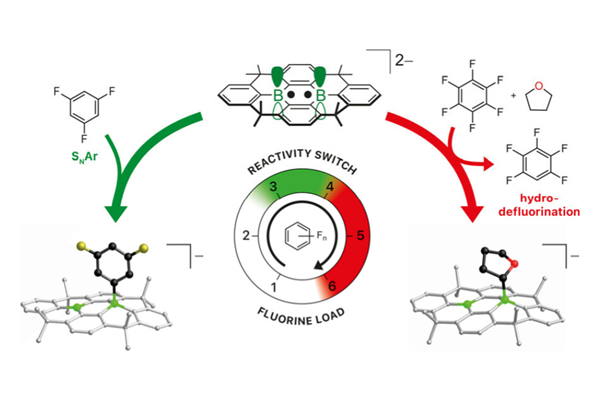
Chemists at Goethe University Frankfurt have created a groundbreaking catalyst capable of rapidly breaking the notoriously strong carbon-fluorine bonds in per- and polyfluorinated substances (PFAS), commonly known as ‘forever chemicals.’ Widely used for their water-, dirt- and oil-repellent properties, PFAS are prised in numerous industries but are also persistent environmental pollutants with potential health risks.
Unlike existing methods relying on expensive and toxic heavy metals such as platinum, palladium, or iridium, this novel catalyst employs two boron atoms embedded within a carbon framework, offering remarkable stability against air and moisture. This unique design enables it to cleave the tough carbon-fluorine bonds within seconds at room temperature.
PFAS are found in countless everyday products — from stain-resistant carpets and outdoor clothing to non-stick cookware and firefighting foams — due to their exceptional durability and resistance to heat and UV light. However, their resilience means they persist in the environment, accumulating in water, soil, plants, and even the human body. Some of the roughly 4,700 known PFAS compounds are linked to cancer and other health concerns.
Professor Matthias Wagner and his team at Goethe University’s Institute of Inorganic and Analytical Chemistry explain that their catalyst efficiently transfers electrons to break these bonds. Currently, alkali metals like lithium serve as the electron source, but efforts are underway to replace these with electrical current, which would streamline and enhance the process.